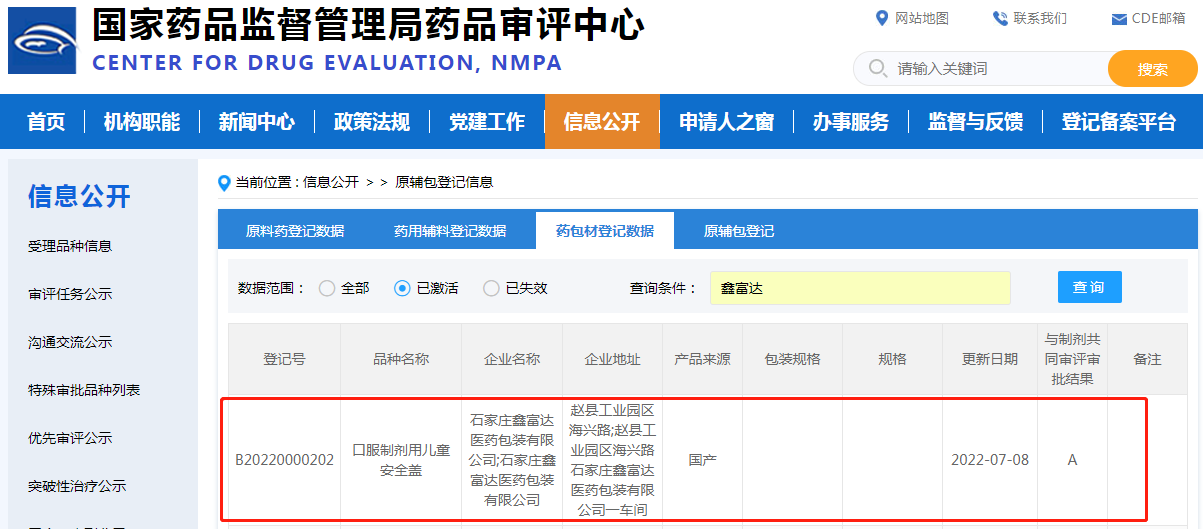
On July 5th, as announced on the website of the Drug Evaluation Center of the State Drug Administration, Shijiazhuang Xinfuda Medical Packaging Co., Ltd.'s child safety cap for oral preparations (registration number: B20220000202) successfully passed the associated review, and the status was changed to A. This means that this product has been affirmed in terms of stability, safety, quality management, policy research and application related to drug submission and approval.
Xinfuda childproof caps for oral preparations adopt a universal 28mm neck, which can be adapted to plastic or glass bottles. Drugs have strict requirements on the sealing of the packaging. In order to achieve a better sealing effect, we have made corresponding special designs on the internal structure of the bottle cap according to the different characteristics of the two materials of plastic and glass. There is an anti-theft ring inside the lid, and the anti-theft ring will fall off automatically after opening, which plays a vigilant role. If it is used for solid drug packaging, the lid can be equipped with a small drug compartment to store the desiccant to absorb the moisture inside the package and maintain the stability of the drug.

The quality of pharmaceutical packaging materials is crucial to the safety, effectiveness, and stability of drugs. Since my country began to implement the associated review system for pharmaceutical packaging materials and drugs, Xinfuda has actively connected with pharmaceutical companies, combining years of basic material research Technology and production experience, demonstration and design of raw material formulations, product structures, molds, and production equipment for pharmaceutical packaging materials, and in-depth and systematic research on quality standards, process verification, compatibility, and stability inspections. Up to now, 14 products including "child safety caps for oral preparations", "cycloolefin polymer pharmaceutical plastic vials (COP vials)" and "oral solid pharmaceutical high-density polyethylene bottles" have been associated with preparations The review was successful.
In the future, we will continue to give full play to our own technological advantages, carry out in-depth exchanges and cooperation with pharmaceutical companies, and jointly promote the high-quality development of the pharmaceutical industry.
Copyright © Shijiazhuang Xinfuda Medical Packaging Co., Ltd. All Rights
MAKE AN ENQUIRY