In the contemporary era of rapid development of medical technology, important changes are also taking place in the way of drug delivery. Disposable Intranasal Atomization Device is a new type of drug delivery device, which atomizes the drug and acts on the whole body through the nasal mucosa. But not all drugs are suitable for this method of administration, and it also has certain requirements for drugs.
Disposable Intranasal Atomization Device
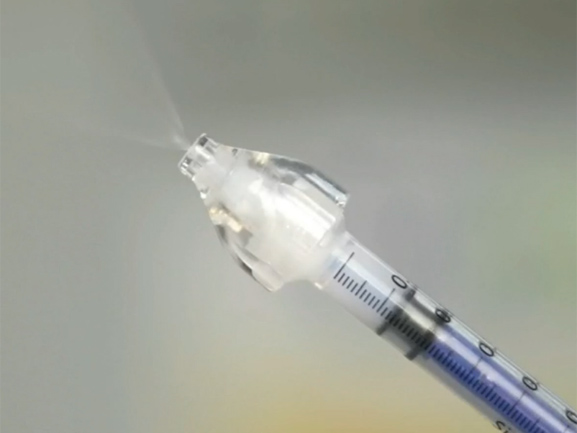
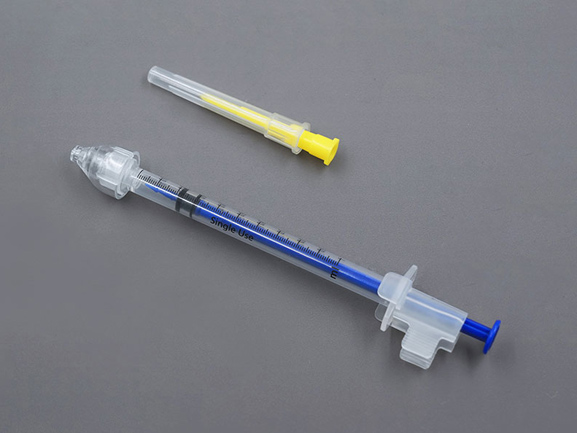
Compared with injection administration, nasal administration is painless, easy to use, and does not require injection. It avoids the first pass of metabolism and improves bioavailability. It can directly reach the cerebrospinal fluid, with good compliance, and is suitable for any patient. However, this method of administration has certain requirements on the drug, and some drugs are not concentrated enough to achieve the ideal administration volume, which is not suitable for nasal administration.
Generally speaking, drugs with small molecular weight, simple structure, and lipophilicity can easily penetrate the membrane, and the concentration of the drug solution is close to the physiological pH value of the human body. If the drug is concentrated to a reasonable volume, nasal administration can achieve better absorption and high bioavailability without flowing out from the nostrils, such as peptides, vaccines and other drugs. The ideal volume of the drug applied to one nostril of the Disposable Intranasal Atomization Device is 0.25ml to 0.3ml. In clinical application, the doctor will use a volume of 1ml for each nostril.

Medical device registration certificate
In conclusion, nasal administration is a simple, safe and convenient new way of administration. It should be noted that if you have allergic rhinitis, nasal mucosal edema, and a lot of runny nose, you should blow your nose and then use the nasal cavity applicator to administer the drug, so as not to affect the absorption of the drug.
Copyright © Shijiazhuang Xinfuda Medical Packaging Co., Ltd. All Rights
MAKE AN ENQUIRY