The official website of the Center for Drug Evaluation of the State Drug Administration shows that the approval status of the CDE number of Xinfuda cyclic olefin polymer pharmaceutical plastic bottle (COP bottle) and the preparation has been successfully activated, from "I" to "A". It means that Xinfuda's COP vials have been recognized in terms of product stability, safety research, production quality management, policy research and application related to drug approval.
In August 2016, in order to fundamentally guarantee and improve the quality of registered drugs, the former State Food and Drug Administration launched the associated review of pharmaceutical packaging materials, pharmaceutical excipients and drugs. After the policy was promulgated, the company joined hands with pharmaceutical companies to actively implement the implementation of the new policy based on market demand and product positioning. As of January 10, 2022, Xinfuda has 13 types of drug packaging and preparations, including COP vial, with the approval status of "A".
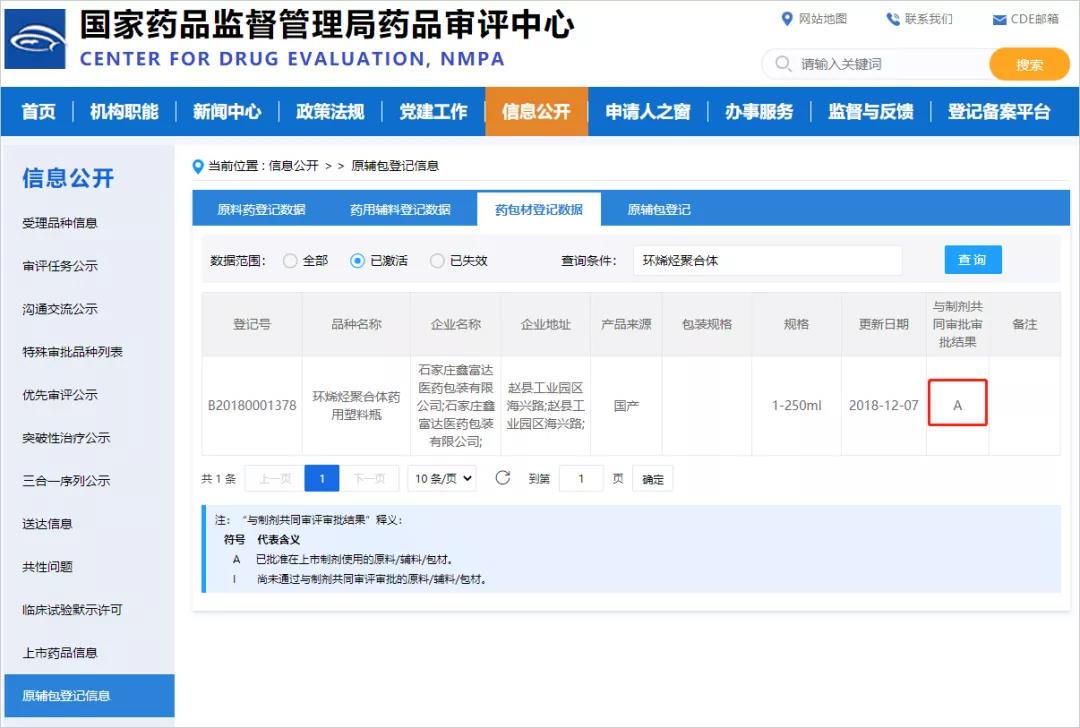
Cyclic olefin polymer medical plastic bottle (COP bottle) is a flagship product of Xinfuda, which is mainly used for the packaging of high-end drugs. Thanks to the good characteristics of the raw materials, the COP bottle can still maintain good stability in the environment of -196°C—+121°C, can withstand drugs with a pH value greater than 10, and is not easy to discolor after irradiation sterilization. Classes and other drugs in special environments can also ensure their safety. Xinfuda is also the first pharmaceutical packaging material manufacturer in China to achieve mass production of COP bottles.
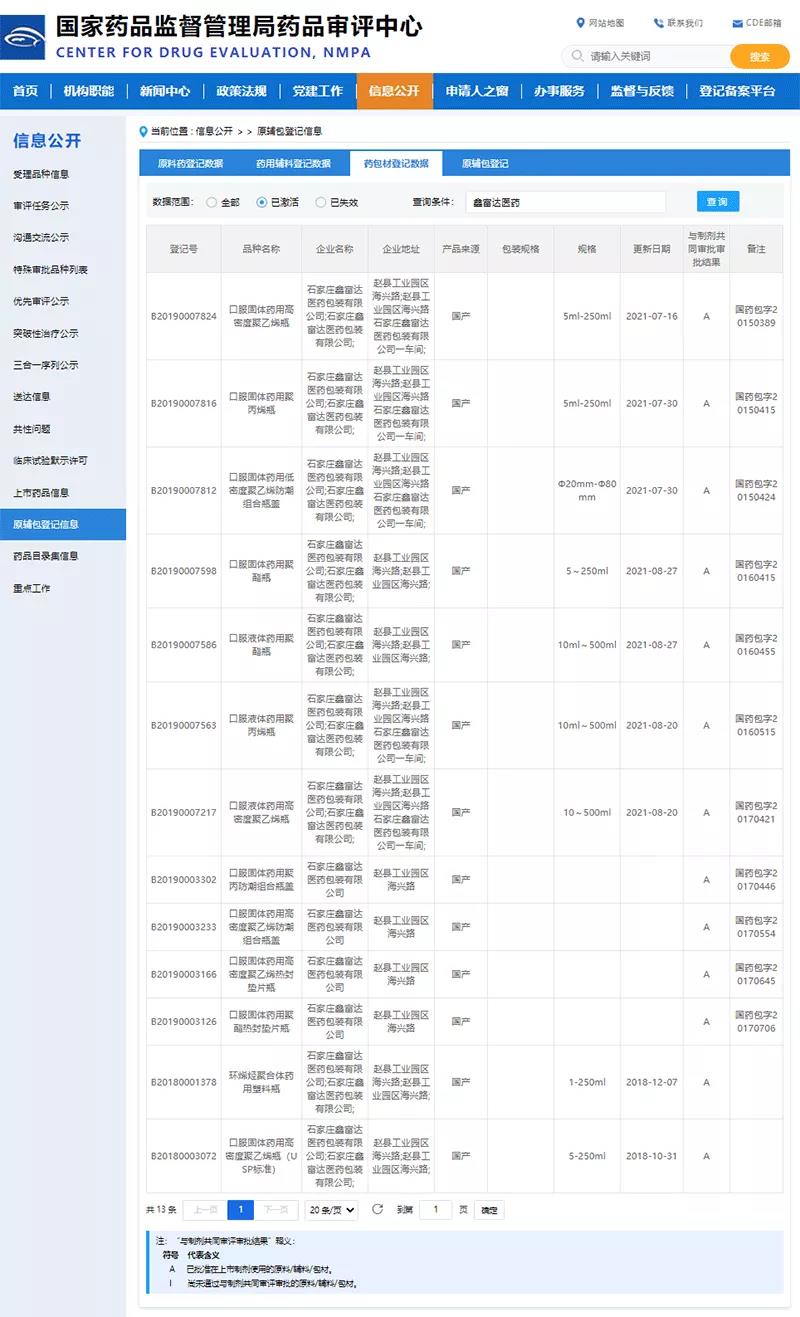
As a high-end drug packaging, the COP vial has passed the associated approval with the preparation, which will provide a better reference for the later selection of drugs in this packaging, reduce the obstacles to approval, and shorten the launch cycle of new drugs. In the future, Xinfuda will continue to strengthen the cooperative research and development with pharmaceutical companies. On the premise of ensuring the safety, effectiveness and quality of the drug, it will develop a pharmaceutical packaging material that meets the storage requirements of the preparation product according to the characteristics of the drug itself, so as to better assist the drug. The company's new drug research and development work.
Copyright © Shijiazhuang Xinfuda Medical Packaging Co., Ltd. All Rights
MAKE AN ENQUIRY